MDI Therapeutics Initiates Phase 1 Clinical Study of MDI-2517
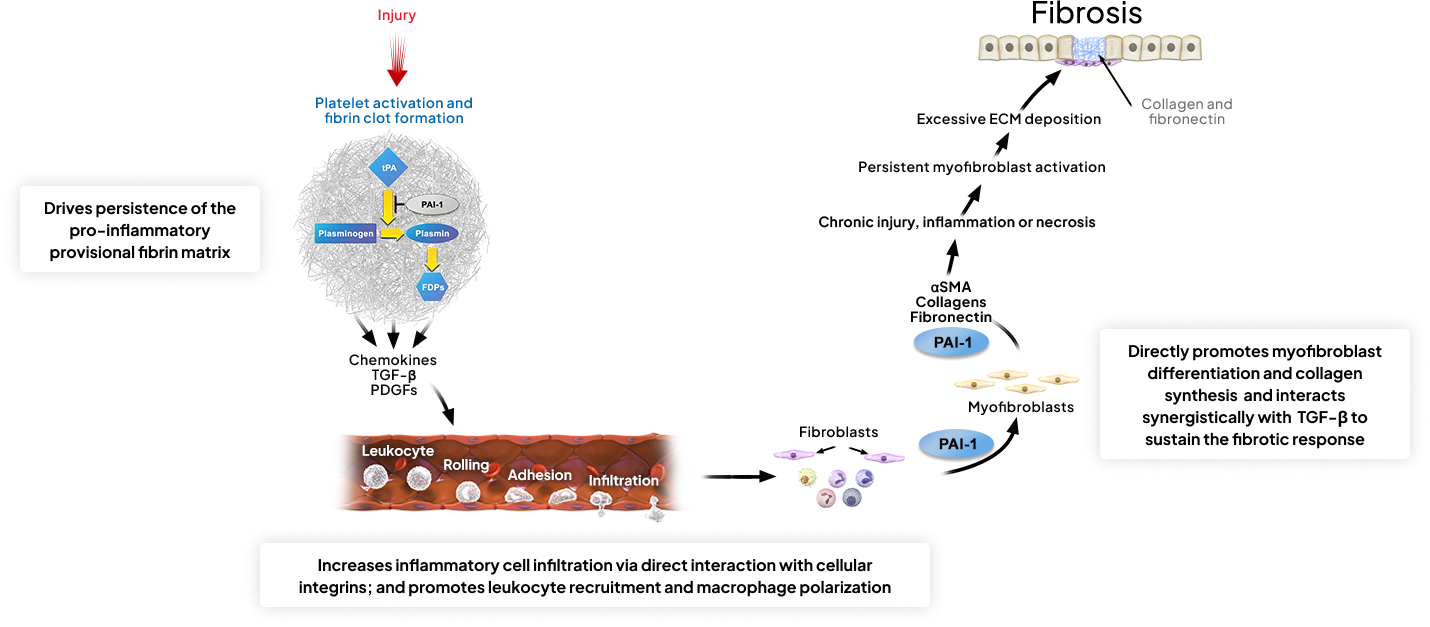
NOVI, MI — June 10, 2024 — MDI Therapeutics, Inc., a pharmaceutical company developing novel therapies for the treatment of fibrosis and fibroproliferative diseases, today announced that it has dosed the first participants in a Phase 1 clinical study evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of MDI-2517, a potent small molecule inhibitor of plasminogen activator inhibitor 1 (PAI-1).
Read More